GUI and module system
Usage
Start the gui by typing instamatic in the command line.
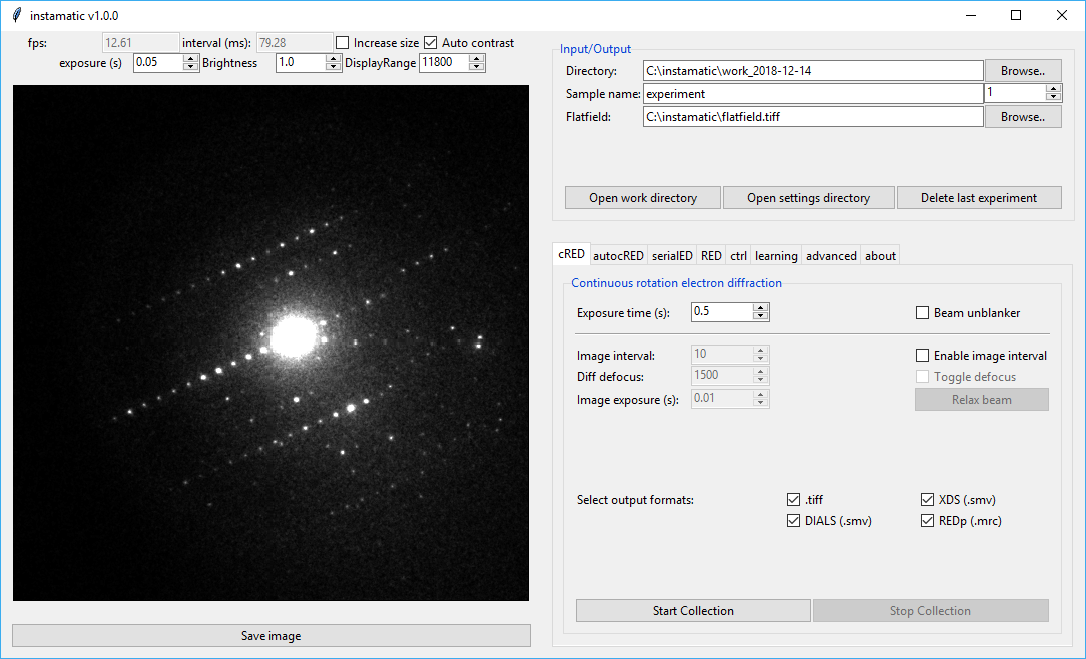
On the left side, a live view of the camera will be shown if the camera can be streamed, such as the Timepix. On the top, there are some fields to change the output of the view. The exposure time for the live view, brightness, and display range can be changed here. Pressing the Save Image button will dump the current frame to a tiff file in the active directory.
On the right side, there is a pane for file i/o, and the available modules for data collection.
Each of the modules can be turned off (except the io module) in the settings.yaml.
Data i/o
Module: io
This panel deals with input and output of the experimental data.
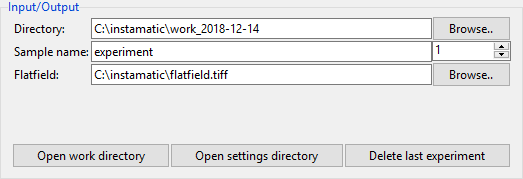
- Directory
- Root directory to work in. By default this is
C:/instamatic/$date/ - Sample name and number
- This determines the subdirectory where experimental data are stored. The number is automatically incremented when a new experiment is started. Data are never overwritten.
- Flatfield
- Here the path to the flatfield image can be specified. The default
value is read from
config/settings.yamland can be left blank if no flatfield should be used. - Open work directory
- Open the current work directory, which is a combination of the root
directory, sample name, and experiment number. In this case
C:/instamatic/work_2017-11-19/experiment_1. All experimental data for the current experiment will be saved here. - Open config directory
- By default
%APPDATA%/instamatic/. The configuration files for the microscope, camera, and calibration files go here. - Delete last experiment
- Sometimes, a data collection will go wrong... Pressing this button will mark the last experiment directy for deletion. It will not actually delete anything, but you will at least know which ones to delete afterwards :-)
Console
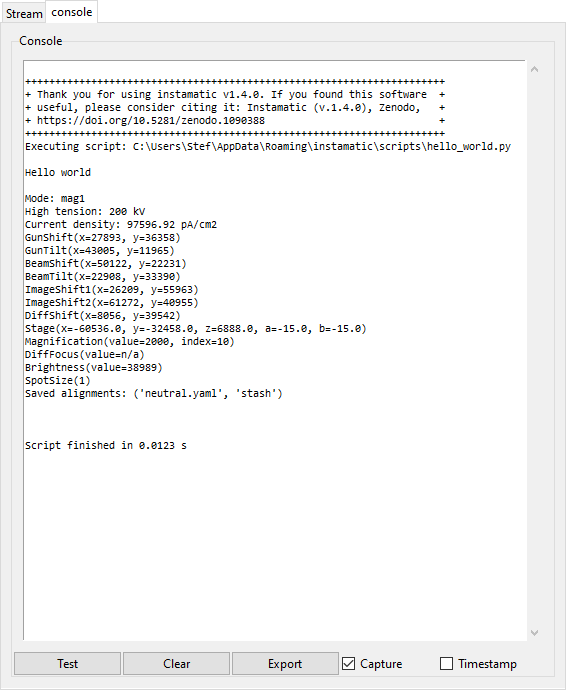
Module: console
If enabled, this panel logs the console output.
- Test
- Print a test message.
- Clear
- Clears the console.
- Export
- Opens a file browser to export the console output to a text file
- Capture
- Check this toggle to redirect the output from
stdout. - Timestamp
- Check this toggle to prepend timestamps to each message.
SerialED data collection
Module: sed
Serial electron diffraction (serialED) is a technique to collect diffraction data on a large number of crystals. One diffraction pattern per crystal is collected. These can then be combined for structure determination, or used for screening/phase analysis.
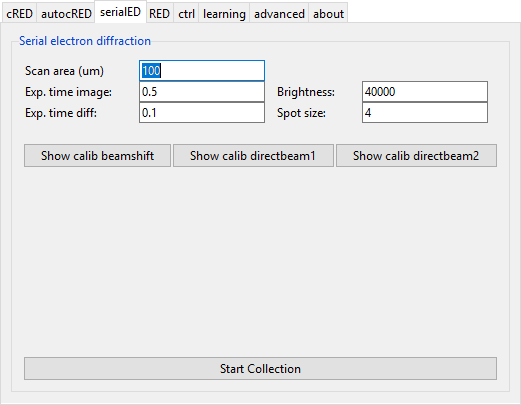
Data collection can be started from the ‘serialED’ tab by pressing the ‘Start Collection’ button. Follow the instructions in the terminal to setup and calibrate the experiment.
- Scan area
- Radius for the area to scan area for crystals (in micrometer).
- Exp. time image
- Exposure time for images.
- Exp. time diff
- Exposure time for diffraction pattern.
- Brightness
- Default value for the brightness of the focused beam.
- Spot size
- Spot size to use.
cRED data collection
Module: cred
Continuous RED (cRED) data collection can be started from the cRED tab.
The data collection procedure can be initiated by pressing ‘Start Collection’. The program will then wait for the rotation to start. The moment the pedal is pressed to start the rotation, the program will start the data collection with the specified options. Do not release your foot until after you press ‘Stop Collection’. This will signal the program to stop data collection, and write the images. Images are written in TIFF, MRC, and SMV format. Input files for REDp (.ed3d) and XDS (XDS.INP) are also written.
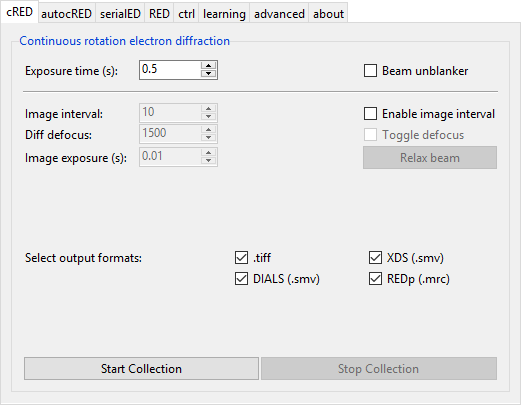
- Exposure time
- change the data collection time for each image.
- Beam unblanker
- If this option is selected, the beam will be automatically unblanked when data collection starts, and blanked after data collection has finished (i.e. after ‘Stop Collection’ has been pressed)
Image interval
With this feature, an image of the crystal will be shown every N frames. This is useful to control the position of the crystal in the beam for more reliable and reproducable data collections. This is achieved by applying a small defocus (diffraction focus) to every Nth image. a small defocus of the diffraction focus. If the defocus is large enough, this will show a view of the crystal in the aperture.
- Enable Image interval
- This option will enable the image interval.
- Image interval
- Change the interval at which the image will be defocused. For example, if the value is 10, then every 10th image will be defocused.
- Diff. defocus
- This is the defocus value to apply. It is better not to make this value too large, because the larger the difference with the proper diffraction focus, the longer the lenses need to recover. The microscope has to switch to the defocus value, take an image, and back within the time it takes to collect a single image (i.e. 0.5 s in this example).
- Toggle defocus
- This toggle applies the defocus value, which is used for checking. It does not affect the data collection.
Other specific cRED modules: cred_fei, cred_tvips, autocred
RED data collection
Module: red
Simple module to collect electron diffraction using discrete rotation steps.
Pressing Start Collection will collect a series of frames with the given tilt step and range. The data collection is then paused, enabling the user to recenter the crystal or finalize the data collection. The program will automatically switch to diffraction mode if it is in image mode, and go back to image mode afterwards. Pressing Continue will continue from the current point, and collect another series of frames with the given tilt range. Pressing Finalize will, not unsurprisingly, finalize the data collection, save the data, and write any input files.
- Exposure time
- The exposure time for each frame in seconds
- Tilt range
- The total tilt range for the next sequence in degrees
- Step size
- The step size in degrees
Data are output to .tiff and .mrc, including input files to read the data in PETS and REDp.
Machine learning
Module: machine_learning
Running instamatic.learn on a series of diffraction patterns collected by SerialED will use a deep convoluted neural network to classify the images in 'good' and 'bad' quality, i.e. those with nice looking diffraction spots are separted from those with powder rings, no signal, lots of overlapping crystals.
The data can be loaded into this tab, to navigate back to the selected crystals.
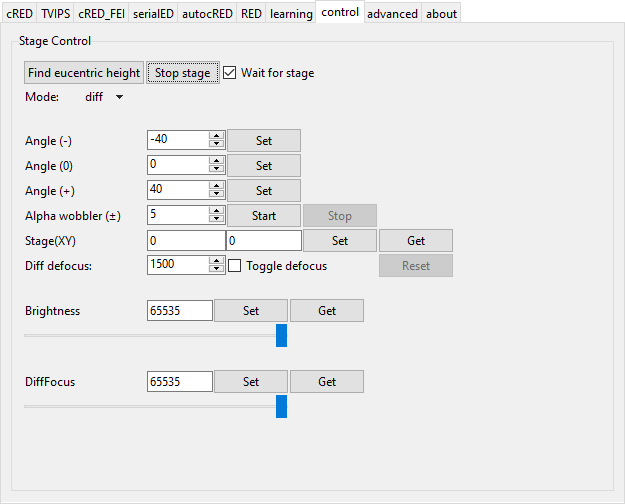
Control
Module: ctrl
This module allows some control over the microscope from the GUI and is updated as needed. At the moment, controls to control the stage position have been implemented, as well as a basic alpha wobbler which will rock the stage back and forth to find the eucentric height for rotation.
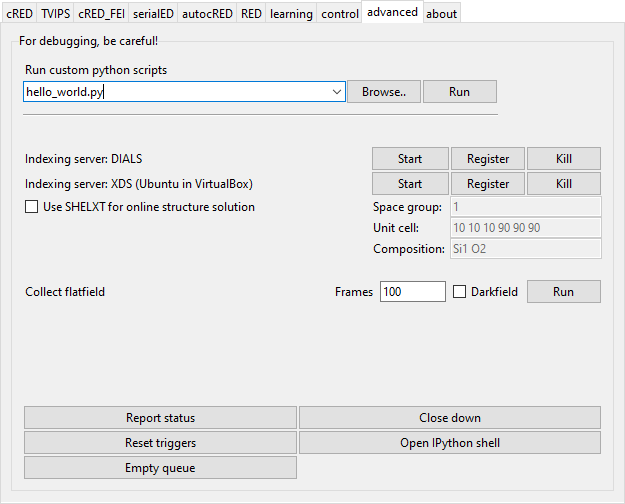
Advanced
This module gives access to several debugging functions, as well as more advanced features.
It also gives the option to run custom python scripts from the GUI. The scripts give access to the ctrl variable, an instance of TEMController. The scripts are picked up from the directory %APPDATA/Instamatic/scripts or as defined by the Instamatic environment variable.
It is also possible to associate an indexing server with the data collection (currently cred, autocred), which will attempt to index the data immediately after data collection
There are also functions to collect a flatfield image.
Report status will report the current status of the microscope to the command line.
Close down will close down the microscope. It is associated to the script close_down.py in the scripts directory. It is meant to put the microscope in a state so that the sample can be removed, this means it will neutralize the sample stage, put down the fluorescence screen, maximize the brightness, etc.
Open IPython shell will open an IPython shell, so that the program state or microscope can be directly interacted with.
Reset triggers and Empty queue are for debugging, and probably redundant at this stage.
Module: debug
About
Module: about